Ụlọ ọrụ mmepụta ihe na-enye ọkwa ụlọ ọrụ mmepụta ihe Glacial Acetic
Site n'ịgbaso ụkpụrụ gị nke "ịdị mma, enyemaka, arụmọrụ na uto", anyị enwetala ntụkwasị obi na otuto site n'aka ndị ahịa obodo na mba ofesi maka ụlọ ọrụ mmepụta ihe na-enye ọkwa ụlọ ọrụ mmepụta ihe, ọnụahịa kachasị elu na asọmpi na-eme ka ngwaahịa anyị nwee ọmarịcha ọkwa ebe niile n'ụwa.
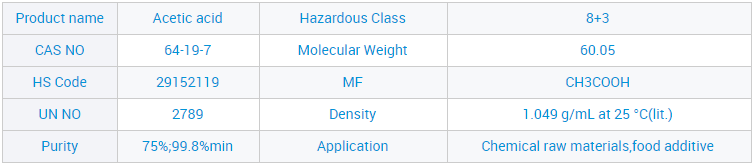
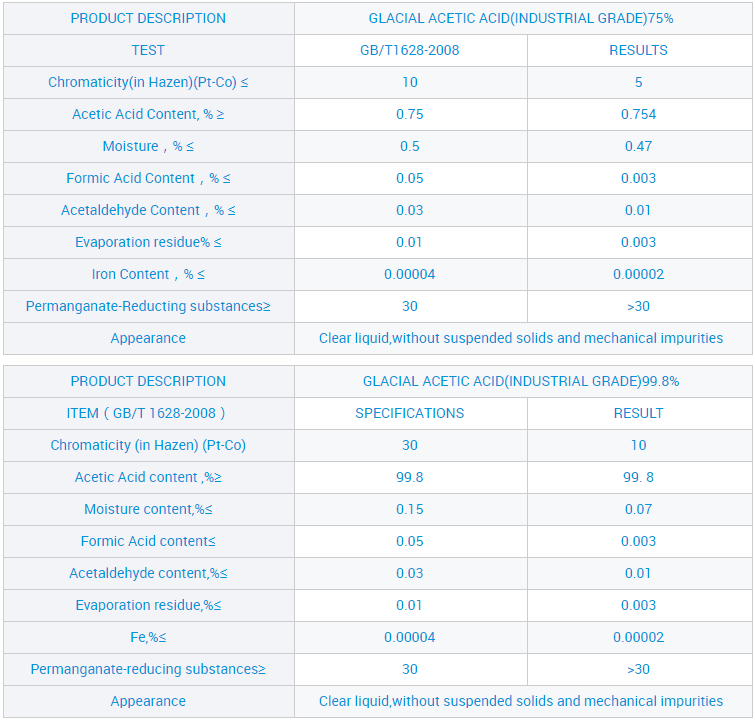
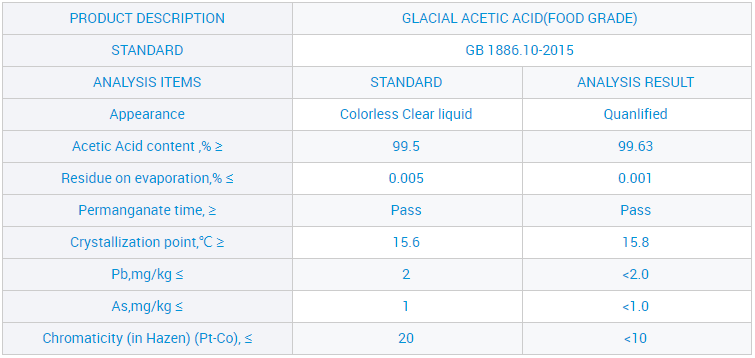
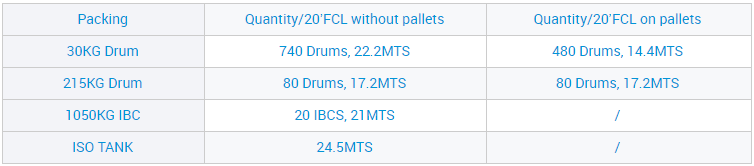
Site n'ịgbaso ụkpụrụ gị nke "ịdị mma, enyemaka, arụmọrụ na uto", anyị enwetala ntụkwasị obi na otuto site n'aka ndị ahịa obodo na mba ofesi maka, dịka ngwọta kachasị elu nke ụlọ ọrụ mmepụta ihe anyị, a nwaleela usoro ngwọta anyị ma nweta asambodo ikike anyị nwere ahụmahụ. Maka paramita ndị ọzọ na nkọwa ndepụta ngwaahịa, jide n'aka na pịa bọtịnụ ahụ iji nweta ozi ndị ọzọ.










 5. Ule:
5. Ule:
1. Asịd Formic:
a. Usoro: Tụọ ihe dị ka g 5 nke ihe nlele ahụ (site na iji nhazi teknụzụ) n'ime beaker 100 ml. Tinye ntapụ abụọ nke ihe ngosi phenolphthalein ma tinye ya na mmiri 2 N NaOH ruo mgbe agba uhie pụtara. Hapụ ya na mmiri ịsa ahụ ruo ọkara olu mbụ. Jụụ ya jụụ, tinye acid na 5 ml nke HCl a gbakọtara agwakọta, wee bufee ya n'ọnụ ọgụgụ n'ime karama iodine 250 ml nke nwere mmiri 50 ml na 50 ml nke mmiri 0.1 N NaBrO. Kpoo ya nke ọma, gwakọta ya nke ọma, ma hapụ ya ka ọ nọrọ ruo nkeji 30. Tinye 15 ml nke mmiri 16.5% KI, 20 ml nke 6 N HCl, na 2 ml nke mmiri 2 starch. Tinye 0.1 N Na₂S₂O₃ mmiri ruo mgbe agba anụnụ anụnụ ahụ ga-apụ n'anya (njedebe). Mee nnwale efu n'otu oge.
b. Ngụkọta: (Millụ efu – ihe nlele ml) × 0.1 (Nhazi) × 0.02301 = gram nke HCOOH
c. Ọkwa Acetic Glacial Rịba ama: Ịkpo ọkụ n'ime mmiri ịsa ahụ mgbe etinyere NaOH bụ ihe e bu n'obi iji gbapụ ma wepụ acetaldehyde, nke ga-ebute nnukwu nsonaazụ. Ọkwa Acetic Glacial.
2. Acetaldehyde:
a. Usoro: Tụọ ihe dị ka 10 g nke glacial acetic acid (site na iji nhazi teknụzụ) n'ime karama iodine 250 ml. Tinye 50 ml mmiri, wee tinye pipette kpọmkwem 10 ml nke 4% NaHSO₃ solution n'ime karama ahụ. Kpochie ya nke ọma, maa jijiji, ma hapụ ya ka ọ nọrọ ruo nkeji 30. Tinye 2 ml nke ihe ngosi starch na titrate na ngwọta 0.1 N Iodine ruo mgbe agba anụnụ anụnụ pụtara (njedebe). Mee nnwale efu n'otu oge.
b. Ngụkọta: (Mil ml - Ihe nlele ml) × 0.1 (Nhazi) × 0.02203 = gram nke CH₃CHO
6. Nkwadebe maka ihe eji eme ihe na reagent:
Ngwọta 0.1 N Sodium Hypobromite (NaBrO): Tinye 2.8 ml nke bromine na mmiri 500 ml. Tinye 100 ml nke 2 N NaOH ma gwakọta ya ruo mgbe ọ ga-agbaze. Gwakọta ya na mmiri na 1000 ml iji nweta ngwọta 0.1 N NaBrO.
6. Nkwadebe maka ihe na-eme ka ... dị n'ime ya dị ka Glacial Acetic Reagent
Ngwọta 0.1 N Sodium Hypobromite (NaBrO): Tinye 2.8 ml nke bromine na mmiri 500 ml. Tinye 100 ml nke 2 N NaOH ma gwakọta ya ruo mgbe ọ ga-agbaze. Gwakọta ya na mmiri na 1000 ml iji nweta ngwọta 0.1 N NaBrO.











